In 1953 GJ Sophian published a book “Toxaemias of Pregnancy” that described his view of preeclampsia based on the findings of the “uterorenal reflex” and the renal corticomedullary “shunt”. It is difficult to find a copy 75 years later. He had become interested in preeclampsia when he read an English translation of Otto Speigelberg’s textbook (1882) that described Speigelberg’s view of the condition. He was also aware of Josep Trueta”s experiments in Oxford (1945-1948) showing evidence of a renal, corticomedullary “shunt” following significant limb injuries in rabbits. The book describes his experiments in blowing up balloons in rabbit uteri and watching sudden changes in renal blood flow. When he interrupted the nerve supply between the uterus and kidney there were no further changes in blood flow. He postulated a “uterorenal reflex” that was triggered by significant increases in uterine “tension” leading to Trueta’s corticomedullary shunt.
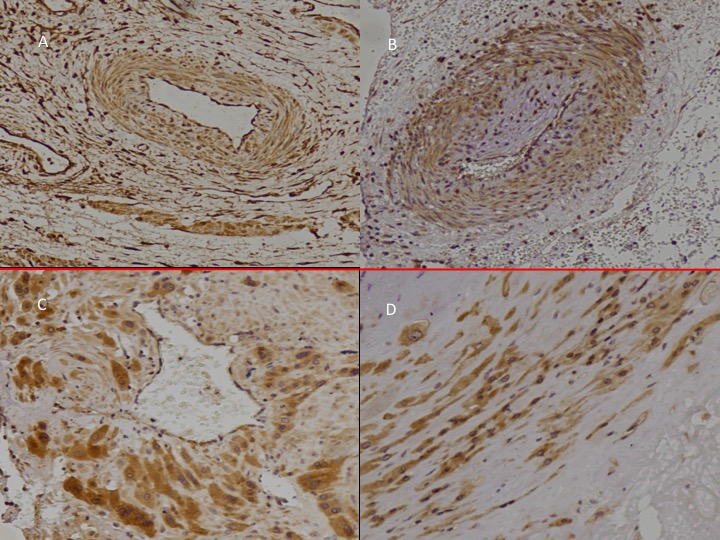
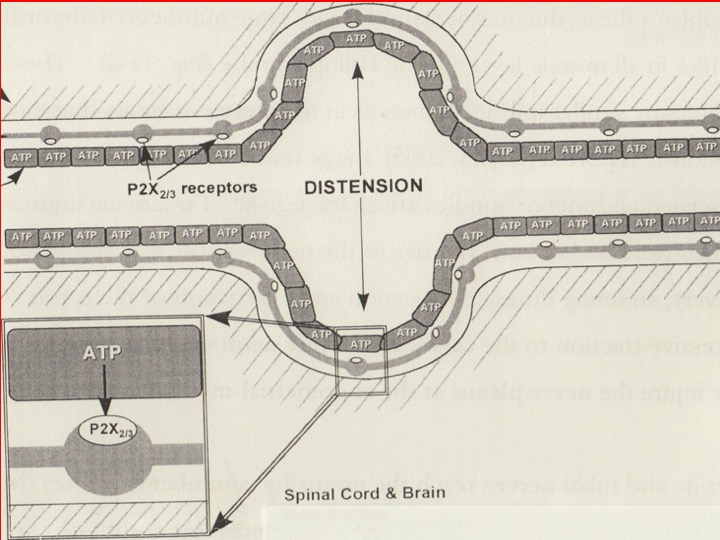
We added the intrauterine P2X3, “stretch” receptors in 2016 to complete the full pathway (Fig.1). We find P2X3 receptors in both arterioles (“early-onset” preeclampsia, Fig 1A-B) and myometrium (“late-onset” preeclampsia. Fig 1C-D). It provides a useful mechanism for understanding the condition. This paper discusses the origins and present position of the “uterine denervation” view.
In this view of preeclampsia there are some important differences from “classic” preeclampsia theory:
- There is no need to postulate “two waves of trophoblast invasion”. A simple uterine denervatory injury is sufficient e.g straining on the toilet, uterine evacuation, etc) as it “induces” P2X3 “stretch” receptors in the uterus
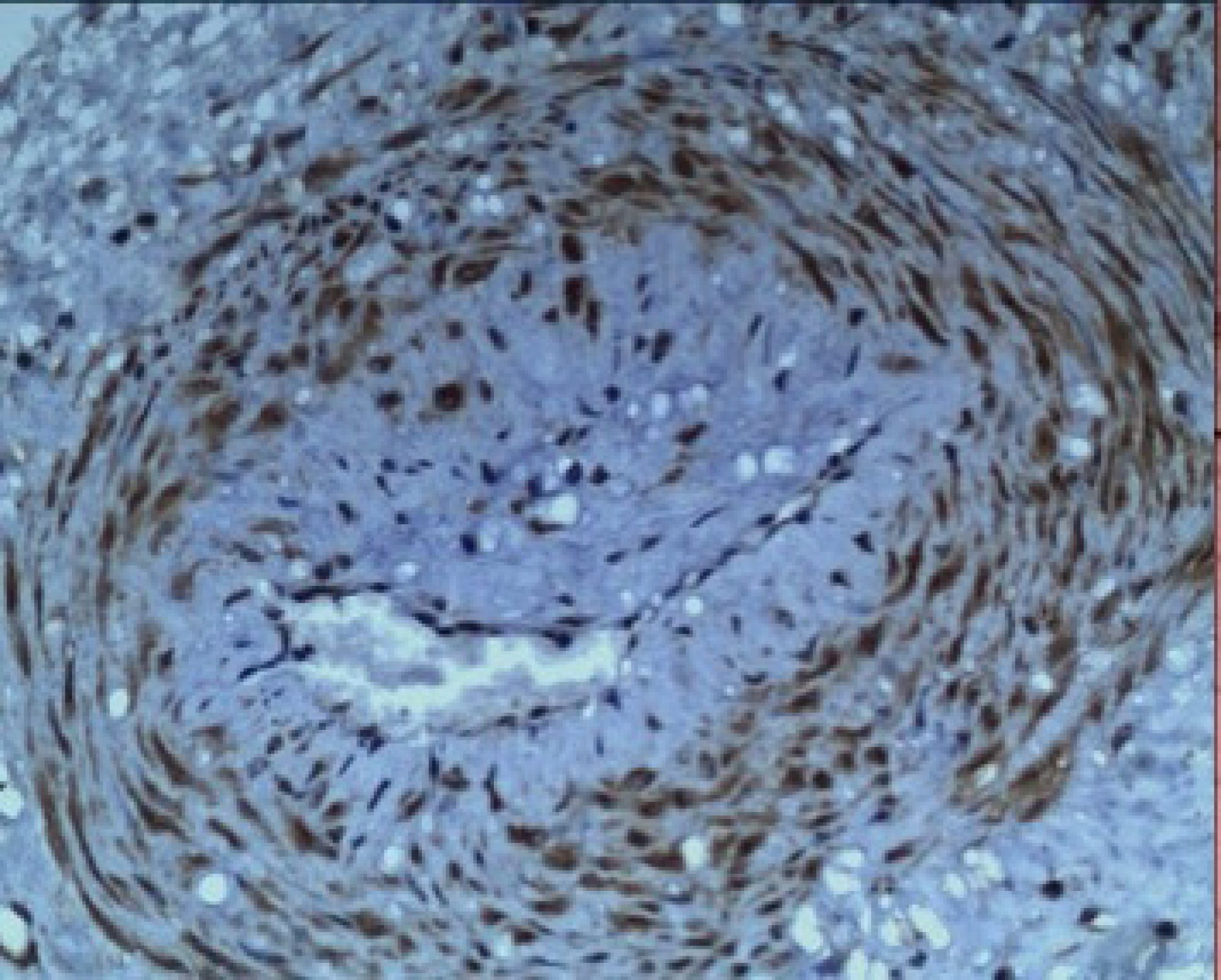
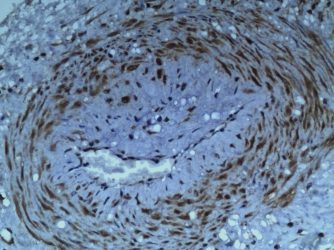
- Few authors have understood the “halo of hyalinisation” around narrowed uterine arterioles in the placenta bed in the “great” obstetric syndromes” (Fig. 2A, pink) , nor made the analogy with the “halo of injured nerves” in the “great gynecologic syndromes” (Fig. 2B, blue).
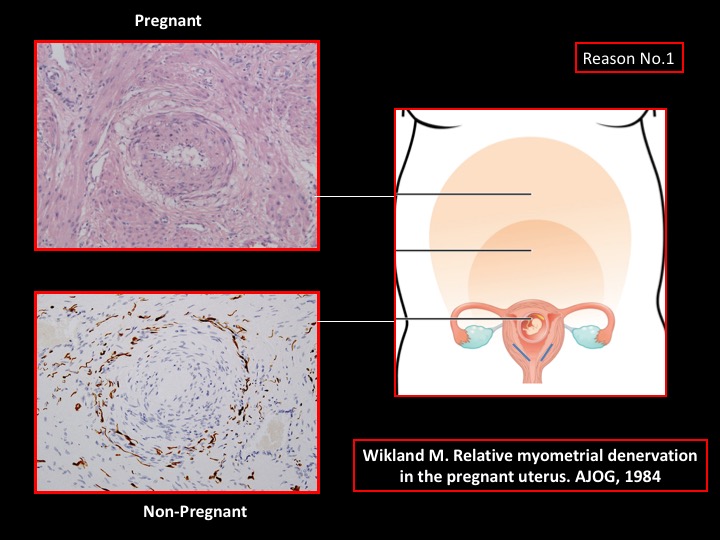
- During pregnancy uterine nerves (blue) are “confined” to the lower uterus and cervix. They do not extend to the placental bed whereas their derivative marker, the “halo of hyalinisation” (pink) betrays their presence by extending to the placenta bed.
- The condition is a result of intrauterine “stretch” rather than any specific “placental lesion”. “Stretch” activates the purinergic, P2X3 receptors, where ATP is the neurotransmitter (Fig 3) that in turn, activates the uterorenal “reflex” and renal, corticomedullary “shunt”. Turning on the RAA system causes hypertension and proteinuria, which resolves as soon as (1) amniotomy, or, (2) delivery takes place.
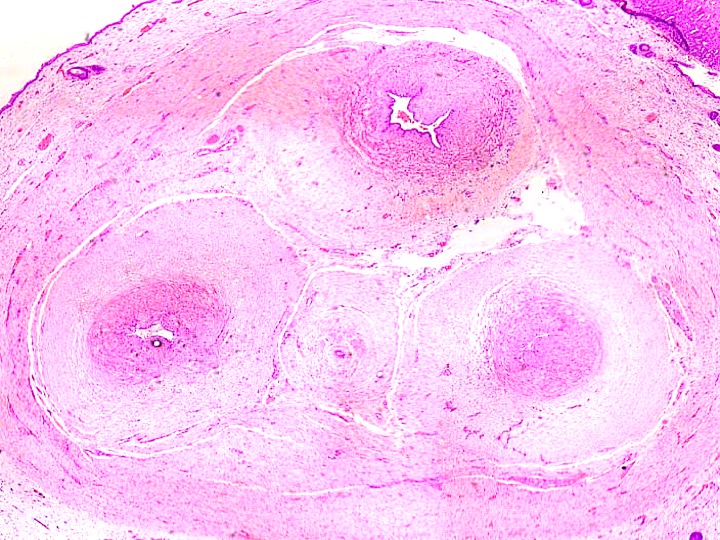
- The mother may “pass” her hypertensive diathesis to her baby possibly by passive transfer of vasopressor substances rather than “fetal programming”. The umbilical vessels demonstrate medial hyperplasia and, in this (severe) example, there is compartmentalisation of Wharton’s jelly and neovascularisation around the periphery of the cord. Medial hyperplasia is similar to that in adult hypertension and is imply the consequence of injuries t vasomotor nerves.
- Hypertension in pregnancy may create further injuries to add to existing maternal renal injuries that promotes early-onset, cardiovascular events e.g stroke and myocardial infarction (age < 50 years).